We know burning fossil fuels is bad because it emits CO2 (a
greenhouse gas)… but did you know that soils emit CO2 too?!
Some of these ‘emissions’ are natural, but poor soil management has significantly increased
them, accelerating climate change. HOWEVER, soils can also absorb
CO2, which could help mitigate climate change. The balance between emission
and absorption determines whether soils are a carbon source (bad) or sink
(good). Let’s investigate …
How much carbon is stored in the soil?
Around 2700Pg (Pg = petagram = 1015g = 1 billion tonnes) of carbon is stored in the soil globally. This consists of ~40% inorganic carbon (SIC), mainly carbonates and ~60% organic carbon (SOC). SOC is more dynamic (more easily lost and restored), so this post will focus on SOC. All SOC values are estimates that have varied widely over recent decades, as shown in the graph below. Total SOC is currently estimated at ~1500Pg.
Around 2700Pg (Pg = petagram = 1015g = 1 billion tonnes) of carbon is stored in the soil globally. This consists of ~40% inorganic carbon (SIC), mainly carbonates and ~60% organic carbon (SOC). SOC is more dynamic (more easily lost and restored), so this post will focus on SOC. All SOC values are estimates that have varied widely over recent decades, as shown in the graph below. Total SOC is currently estimated at ~1500Pg.
 |
|
Fig. 1. Estimates of global soil organic carbon stocks from the
literature through time. Median across all estimates 1460.5 Pg C, range
504–3000 Pg C, n = 27 studies, based on spatially explicit (red; median 1437 Pg
C, range 504–2469.5 Pg C, n = 7) and nonspatially explicit methods (blue;
median 1388.5 Pg C, range 710–3000 Pg C, n = 20). Lines connect minimum and
maximum estimates of soil organic carbon reported by the same study. (Source:
Scharlemann et al., 2014)
|
Is 2700Pg a lot or a little? To give some sense of scale I’ve
placed these values in the context of the 5 main global carbon pools in the diagram
below. (The circles aren’t drawn to scale because the oceanic pool
is comparatively so large).
 |
| Fig. 2 The five major global carbon pools along with the % of total carbon that they contain (diagram drawn by me but based on data from Lal, 2008 and Scharlemann et al., 2014) |
WOW – the oceans vastly overshadow the other four pools! However soils are clearly still significant. They store over 3 times as much as the atmosphere and around 4 times as much as all living organisms. In fact, soils are responsible for most of the biotic storage because ~80-90% of this is land plants.
The SOC budget:
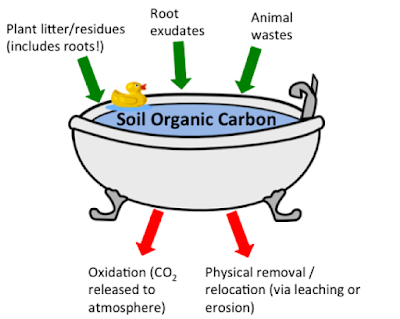
The mass of SOC depends on the balance between inputs and outputs (Fig.3), just as the water level in a bathtub is determined by the flow rate in through the tap and out through the drain. The diagram below shows the main inputs and outputs to the ‘SOC bath’.
The mass of SOC depends on the balance between inputs and outputs (Fig.3), just as the water level in a bathtub is determined by the flow rate in through the tap and out through the drain. The diagram below shows the main inputs and outputs to the ‘SOC bath’.
 |
|
Fig. 4 Soil organic carbon budget represented as water in a bath along
with the main carbon inputs and outputs (diagram made by me).
|
Distribution of SOC:
SOC levels vary globally. The first map below shows large-scale SOC trends that result from differences in climate. The most carbon rich soils are found in cold, often waterlogged, northern regions where oxidation of organic matter is slow. The second map shows carbon stored in the terrestrial biome. Dr Ed Tanner, co-author of these maps, explained that, “if we’re interested in conserving carbon, which we ought to be, we ought to conserve soils in temperate regions, and plants in tropical regions”.
SOC levels vary globally. The first map below shows large-scale SOC trends that result from differences in climate. The most carbon rich soils are found in cold, often waterlogged, northern regions where oxidation of organic matter is slow. The second map shows carbon stored in the terrestrial biome. Dr Ed Tanner, co-author of these maps, explained that, “if we’re interested in conserving carbon, which we ought to be, we ought to conserve soils in temperate regions, and plants in tropical regions”.
Are we losing SOC?
Yes! Cultivated (although not all) soils have lost between 50-75% of their original SOC pool. But remember that only chemical SOC loss increases atmospheric CO2. Depletion of SOC has so far contributed about 78±12 Pg C to the atmosphere. Land-use change has contributed a further 136±55 Pg C. This compares to an estimated 270±30 PgC contributed by fossil fuel combustion.
Yes! Cultivated (although not all) soils have lost between 50-75% of their original SOC pool. But remember that only chemical SOC loss increases atmospheric CO2. Depletion of SOC has so far contributed about 78±12 Pg C to the atmosphere. Land-use change has contributed a further 136±55 Pg C. This compares to an estimated 270±30 PgC contributed by fossil fuel combustion.
 |
|
Fig. 6 The historic CO2 emissions from the pedologic,
biotic and geologic carbon pools into the atmosphere (based on data from Zomer et al., 2017)
|
Why are we losing SOC?
(1) Land use change from forests to agriculture has reduced organic
inputs because the crops that replaced the trees are harvested, not left
as litter when they die.
(2) Conventional ploughing increases the exposure of soil to air, which increases the rate of oxidation. (I had no idea that ploughing was such an issue!).
(2) Conventional ploughing increases the exposure of soil to air, which increases the rate of oxidation. (I had no idea that ploughing was such an issue!).
(3) You may imagine the carbon rich soils of the Northern hemisphere are safe
because these areas are too cold for agriculture... but unfortunately not.
Increased temperatures due to climate change are predicted to increase rates of decomposition and
therefore increase the amount of CO2 they
release, triggering a positive feedback loop.
 |
|
Fig. 7 A farmer tilling the soil in preparation to plant
strawberries in Watsonville, CA (Source: Kip Evans – National Geographic Society)
|
Can't nature counterbalance this?
Increased CO2 = more photosynthesis = more
terrestrial carbon storage… this makes sense but:
(1) there still needs to be land available for this extra plant
growth
(2) a recent paper found that increased atmospheric CO2 levels may also increase soil microbial activity and thus the rate of SOC decomposition. The authors estimate that this would largely neutralise the positive effect of increased photosynthesis, suggesting that ‘nature may not be as efficient at slowing global warming as we previously thought’.
(2) a recent paper found that increased atmospheric CO2 levels may also increase soil microbial activity and thus the rate of SOC decomposition. The authors estimate that this would largely neutralise the positive effect of increased photosynthesis, suggesting that ‘nature may not be as efficient at slowing global warming as we previously thought’.
How can we increase soil carbon?
(1) Reforestation/afforestation
(2) Soil management techniques such as retention of crop residues, cover cropping and no till planting (see video below). A recent paper estimates that altering land management practices on cropland could sequester up to 1.85Pg of carbon per year. This is around 25% of fossil fuel CO2 emissions (~7.5-8Pg yr-1), equal to the transportation sector’s current carbon emissions!!!
(3) There is a limit to sequestering carbon using the above techniques and this is approximately equal to the historic C loss (~80Pg). Adding biochar (i.e. charcoal used for soil amendment) to soils has been proposed as a method of surpassing this limit. Biochar is stable, resistant to microbial degradation and can remain in the soil for millennia. Using data from the World Bank, I calculated that adding ~70 t/ha of biochar to all cultivated soils would sequester enough carbon to return atmospheric CO2 to pre-industrial levels.
 |
|
Fig. 9 A fine textured biochar that could be added
to the soil to help sequester carbon (Source: NOVA – PBS, 2015)
|
(4) Let’s not entirely forget
SIC. When silicate rocks are exposed to air and water they react with CO2
to form innocuous bicarbonates. These are then stored in the soil or washed into the
oceans. For the magnesium-silicate
olivine, the reaction is as follows:
If silicates were mined, milled and spread
over the soil surface it would increase the rate of weathering, remove CO2 from the atmosphere and increase long-term SIC stocks.
 |
| Fig. 10 An olivine (magnesium-silicate) rock (Source: University of Arizona, 2011) |
In conclusion…
Current agricultural practices are causing
soils to emit more CO2 than normal, accelerating climate change. Rising
temperatures may further increase these emissions (particularly on unfarmed
northern soils), creating a positive feedback loop. Changing agricultural
practices could restore much of the SOC that has been lost. Addition of biochar
and accelerated silicate weathering could then increase soil carbon storage
beyond natural levels. Converting soil from a carbon source to a carbon sink
could be a key strategy to mitigating climate change.




Was fascinated by this Becca. I have written a few posts of the role of soil in the carbon cycle recently, specifically on the impact of climate change on heterotrophic respiration and soil respiration (https://jamesandthegiantbeachblog.wordpress.com/2017/02/28/climate-change-and-soil-has-a-carbon-sink-become-a-source-of-carbon/) and the potential of black carbon to sequester carbon (https://blackcarbontheblackdeathofourplanet.blogspot.com/2017/12/it-is-not-all-doom-and-gloom-could-use.html). I was just wondering how you'd come to the ~70t/ha of biochar addition to reduce atmospheric carbon dioxide to pre-industrial levels? I haven't come across a figure in my reading.
ReplyDeleteHi James,
ReplyDeleteThanks for reading. I had a look at the posts you linked – really interesting stuff and glad you’re getting nerdy about soil too!
You won’t have come across the figure in your reading because I calculated it myself based on some data from the World Bank and some simple chemical values. I’ve tried to explain my calculations below:
ppmv = parts per million by volume. In terms of volume…
Current atmospheric CO2 = 400 ppm(v)
Pre-industrial atmospheric CO2 = 260 ppm(v)
Therefore we need to sequester 140 ppm(v) of CO2 to go from current to pre-industrial levels.
I want to calculate the absolute mass of carbon that we need to sequester. Therefore, first I need to convert ppm by volume into ppm by mass.
= (ppmv of CO2 x molecular weight of CO2)/molecular weight of air
= (140 x 44)/29
= 213 ppm(m)
I then need to convert ppm(m), which is a ratio, into an absolute amount.
= ppm(m) of CO2 x weight of atmosphere per km2 x total surface area of the earth km2
= 213 x 10 x 510,100,000
= 1.09 x 10^12 tonnes of CO2
I want the total mass of carbon, not the total mass of CO2 so …
(Total mass of CO2 x atomic weight of C)/molecular weight of CO2
= (1.09 x 10^12 x 12)/44
= 2.96 x10^11 tonnes of carbon
I now need to work out how much carbon we must sequester per unit area of agricultural land.
= total mass of carbon to sequester (tonnes)/ total area of land under agriculture (km2)
= (2.96 x10^11) / 48,390,409
= 6116.9 tonnes/km2
To convert from km2 to hectares I divided this value by 100 so..
=61.169 t/ha
This means we need to sequester ~60 tonnes of carbon per hectare of agricultural land to return atmospheric CO2 concentration to pre-industrial levels.
However, charcoal/biochar is not 100% carbon. Its carbon content does vary, but for this calculation I assumed that biochar is 90% carbon… therefore
61.169 / 0.9 = 67.96 t/ha of biochar
…which I then rounded up to 70t/ha.
And there you have the value of 70 tonnes per hectare of biochar needed to return the atmospheric CO2 concentration to pre-industrial levels.