“Turning stones into bread”
Fritz Haber
Nutrient depletion is the second main cause of global soil degradation (see my second post). Fertilizers can restore, maintain and enhance chemical soil quality depending on the quantities in which they are added. They can restore/maintain productivity by replacing the nutrients lost through harvesting. They can then enhance productivity if nutrients are added beyond natural baseline levels (although this only translates into increased plant growth if these nutrients were naturally limiting). This post investigates the use of artificial nitrogen fertilizers, covering both their revolutionary agricultural benefits and also their significant environmental threats.
Natural N fixation
Nitrogen
is the most important nutrient for plant growth. Yet, it is also the most naturally limiting. Atmospheric nitrogen (N2) is abundant,
comprising 78% of the air we breathe, but unavailable to plants. There are only two ways that atmospheric nitrogen is naturally
converted into plant available nitrogen (i.e. fixed): (1) via
lightning or (2) via specialized ‘Rhizobium’ bacteria found in the root nodules of certain
‘leguminous’ plants. No wonder it’s in short supply!
 |
Fig. 1 Elemental nitrogen: essential to life
|
Artificial N fixation
In 1909 Fritz Haber invented a process (outlined in Fig. 3 below), which
allowed humans to artificially fix atmospheric nitrogen to produce biologically
available ammonia. In the 1930s, Carl Bosch then developed this process on an
industrial scale and by the 1950s synthetic ammonia fertilizers were being mass-produced all over the world.
 |
Fig. 2 Fritz Haber (left) and Carl Bosch (right)
|
 |
Fig. 3 Diagram showing the Haber-Bosch process for converting
unreactive atmospheric nitrogen into plant available ammonia. (Source: Gulf Coast Environmental Systems)
|
 |
Fig. 4 Trends in human population and nitrogen use throughout the
twentieth century. Of the total world population (solid line), an estimate is
made of the number of people that could be sustained without reactive nitrogen
from the Haber–Bosch process (long dashed line), also expressed as a percentage
of the global population (short dashed line). The recorded increase in average
fertilizer use per hectare of agricultural land (blue symbols) and the increase
in per capita meat production (green symbols) is also shown (Source: Erisman, 2008)
|
The Bad & The Ugly:
Whilst the production of artificial fertilizers has clearly been good for humanity, it has also begun to cause serious environmental problems. Because biologically available nitrogen is so rare in nature, ecosystems are highly sensitive to it. Nitrogenous fertilizers (that have not yet been incorporated into crop plants) can wash off
fields and into watercourses, where they stimulate blooms of algae. These
blooms trigger a process called ‘eutrophication’, which depletes the water column of oxygen to a point where no life is possible, as shown in the diagram below.
 |
Fig. 5 Fertilizer washes into a water body causing eutrophication.
At the end of the eutrophication process the oxygen levels in the water are so
low that no life can exist. (Source: BBC Bitesize)
|
 |
Fig. 6 Map showing location of major dead zones (where marine life
cannot survive) caused by in washing of excess reactive nitrogen (Source: NASA, 2017)
|
The Nitrogen Boundary:
In 2009, a group of scientists proposed 9 'planetary boundaries' (PBs) that humanity must not cross if we are to continue to support life and our
civilization. These scientists deemed the side-effects of increased nitrogen-fixation to be significant enough for nitrogen to have it's own boundary. The PB framework was updated in 2015 and the original nitrogen boundary amalgamated intro a more holistic 'biogeochemical flows' boundary. Nevertheless, both frameworks identify that we are already WAY beyond the safe zone for nitrogen use, as shown in the diagram below. The flow of human-fixed nitrogen must be reduced by about a third of its current value
(from ~150 million tonnes to ~62 million tonnes per year) to return to the
‘safe zone’.
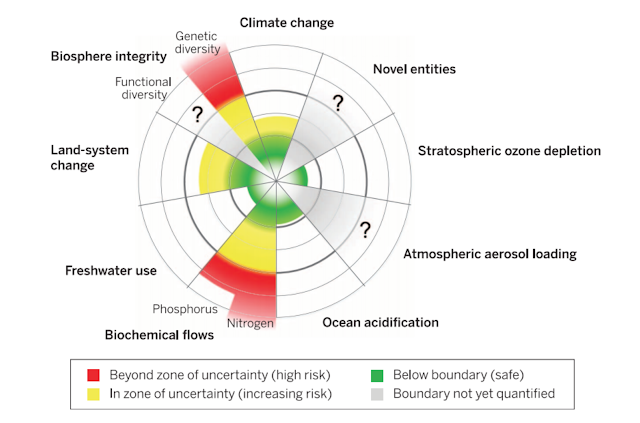 |
Fig. 7 Current status of the control variables for seven of
the planetary boundaries. The green zone is the safe operating space, the
yellow represents the zone of uncertainty (increasing risk), and the red is a
high-risk zone. The planetary boundary itself lies at the intersection of the
green and yellow zones (Source: Steffen et al., 2015)
|
A question of distribution?
The transgression of the global nitrogen boundary is mainly due to a few agricultural regions where fertilizer application rates are so high that nitrogen is often actually in excess. Perhaps a redistribution of nitrogen from these areas to areas where the soil is still nitrogen-poor could maintain global crop production whilst reducing the need for nitrogen-fixation. Unfortunately, this is not as simple as it sounds because artificial fertilizers are expensive and many of the regions with low usage (e.g. Sub-Saharan Africa) are unable to afford them. Therefore, the next few posts investigate other ways we could reduce artificial nitrogen-fixation.
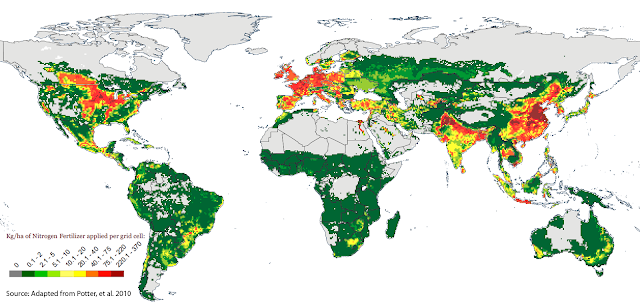 |
Fig. 8 Map
of global nitrogen fertilizer application in Kg/ha. (Source: adapted from Potter
et al., 2010)
|










